Rentiapril racemate
CAS No. 72679-47-1
Rentiapril racemate( —— )
Catalog No. M33478 CAS No. 72679-47-1
Rentiapril racemate (SA-446 racemate) is the racemic form of Rentiapril, exhibiting anti-inflammatory properties and potential applications in glaucoma research.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 182 | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameRentiapril racemate
-
NoteResearch use only, not for human use.
-
Brief DescriptionRentiapril racemate (SA-446 racemate) is the racemic form of Rentiapril, exhibiting anti-inflammatory properties and potential applications in glaucoma research.
-
DescriptionRentiapril racemate (SA-446 racemate) is the racemate of Rentiapril. Rentiapril is an angiotensin converting enzyme (ACE) inhibitor.
-
In Vitro——
-
In VivoA three-months toxicity study of an angiotensin converting enzyme (ACE) inhibitor, Rentiapril (CAS 80830-42-8), is performed in Sprague-Dawley rats by oral administration. The dose levels of 0, 30, 125, 500 and 1000 mg/kg are tested in both sexes, in which each experimental group comprised 10 rats. Another ACE inhibitor, captopril, is used as a reference compound. Rentiapril at the highest dose of 1000 mg/kg causes low food consumption and death of some animals with signs of bloody feces and anemia. In males and females receiving 500 and 1000 mg/kg, there are low body weight gain, increases in water intake, urine volume and serum BUN level, and decreases in levels of various erythrocytic parameters. Kidney weight is increased dose-dependently in both sexes. Histopathologically, renal changes in the 500 and 1000 mg/kg groups consist of proximal tubular degeneration, juxtaglomerular cell hyperplasia and interstitial cell infiltration. Similar, but mild, changes in proximal tubules are present in the female 125 mg/kg group. Dead animals from the highest dose groups further show gastrointestinal hemorrhagic erosion and/or ulcer, decrease bone marrow erythropoiesis and hepatocytic vacuolar degeneration. There is no pathological alteration in rats from other Rentiapril-treated groups, as well as in controls. These results indicate that the no-effect dose of Rentiapril in rats by three months oral administration is 30 mg/kg in female and 125 mg/kg in male.
-
Synonyms——
-
PathwayMetabolic Enzyme/Protease
-
TargetACE
-
RecptorAngiotensin-converting Enzyme (ACE)
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number72679-47-1
-
Formula Weight313.39
-
Molecular FormulaC13H15NO4S2
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (319.09 mM; Ultrasonic )
-
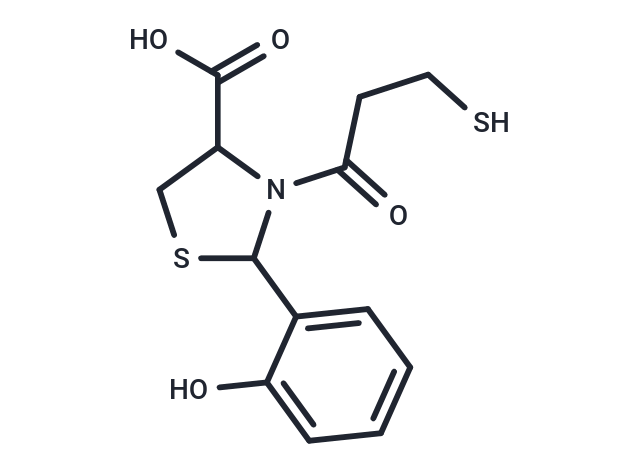
SMILESOC(=O)C1CSC(N1C(=O)CCS)c1ccccc1O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Takase K, et al. Toxicity study of the angiotensin converting enzyme inhibitor rentiapril in rats. Arzneimittelforschung. 1995 Jan;45(1):15-8.?
molnova catalog



related products
-
H-Ile-Pro-Pro-OH
H-Ile-Pro-Pro-OH, a milk-derived peptide, inhibits angiotensin-converting enzyme (ACE) with an IC50 of 5 μM.
-
Rentiapril racemate
Rentiapril racemate (SA-446 racemate) is the racemic form of Rentiapril, exhibiting anti-inflammatory properties and potential applications in glaucoma research.
-
Gemopatrilat
A potent, orally available, dual ACE-neutral endopeptidase (vasopeptidase) inhibitor with IC50 of 12 and 63 nM, respectively.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


